
Gallium therefore has three valence electrons. The 4s and 4p electrons can be lost in a chemical reaction, but not the electrons in the filled 3d subshell. Gallium has the following electron configuration. Lithium has a single electron in the second principal energy level, and so we say that lithium has one valence electron. How many valence electrons are in lithium? Therefore, the valence electrons of krypton are eight. The electron configuration of krypton shows that the last shell of krypton has eight(4s 2 4p 6) electrons. The total number of electrons in a valence shell is called a valence electron. Furthermore, the number of electrons orbiting around the nucleus is 36 also. So, the number of protons in the nucleus of an atom of Krypton is 36. How many subatomic particles are in krypton?Įxample: The element Krypton has the atomic number 36.

Properties of Krypton-84 Isotope: Properties of Krypton-84 Isotope:

#Krypton number of neutrons how to
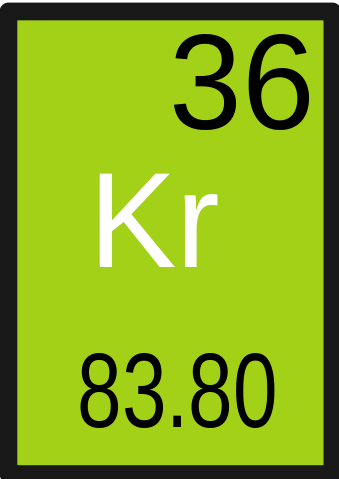
How to Write the Formula for Krypton hexafluoride – YouTube What is the mass of krypton in grams?Īnd krypton, Kr, with atomic number 36, has a molar mass of 83.798 g/mol. Then multiply the moles Kr by the molar mass of Kr, which is 83.798 g/mol (atomic weight in grams/mole). How do you find the mass of krypton?Įxplanation: You first need to determine the moles of krypton (Kr) using the ideal gas law. The mass number of common krypton is 84, and its atomic number is 36. How many protons and neutrons does krypton have?
#Krypton number of neutrons full
An atom of Krypton has a full outer shell so it is inert. Krypton has 36 protons and 48 neutrons in its nucleus giving it an Atomic Number of 36 and an atomic mass of 84. What isotope has 36 protons and 48 neutrons? They decay into other particles within days, giving off more radiation. Krypton-92 and Barium-141 are both unstable. Kr-85, the most stable radioactive isotope, has a half-life of 10.76 years and it is produced in fission reactors. 6 of them are natural, 5 of which are stable. Generally krypton is not isolated, but it is used with other inert gases in fluorescent lamps. This becomes relevant in nuclear chemistry, where sometimes, you have to balance nuclear equations with respect to the mass numbers (nucleon numbers). In the case of Krypton-84, this means that you have 84 nucleons, where 36 of these are protons, and the remaining 48 are neutrons. Atomic NumberĮxplanation: Since Kr or Krypton is a noble gas, it has a full valence shell or octet of 8 electrons. Krypton atoms have 36 electrons and the electronic shell structure is with Atomic Term Symbol (Quantum Numbers) 1S 0. The other inert gases including argon and xenon also have full outer shells with eight electrons. This is one of the happy elements and has an electron configuration of 2-8-18-8. Since krypton is in the far right row of the periodic table, its outermost shell is full with eight electrons. Krypton ( Kr), chemical element, a rare gas of Group 18 (noble gases) of the periodic table, which forms relatively few chemical compounds. How do you find the number of neutrons in krypton? 36Ģ007 Schools Wikipedia Selection.

Since krypton ‘s atomic number is 36, Kr has 36 protons. What is the number of electrons in Krypton? It has a melting point of -157°c and a boiling point of -153°c.Advertisements. It is located in Group 18 as a non metal gas which is odourless and colourless. Krypton’s main uses are in photography equipment for lighting and in high powered lasers. They named the new gas Krypton, from the Greek ‘kryptos’ meaning ‘hidden.’ Krypton as a gas was used as a scientific length between 19 as the measurement of a metre being defined as 1 650 763.73 wavelengths of krypton-86’s orange-red spectral line. After removing Nitrogen and Oxygen by heating with Copper and Magnesium, they applied a high voltage to the left over gases and produced a yellow and green spectrum, which had never been seen before. Obtaining a sample of air as a liquid they began to evaporate off gases. He had built up a picture of the gases existing in the same group. Ramsey had a track record of discovering Noble gases having discovered helium and argon. It was discovered in 1898 by Scottish chemist William Ramsey and his assistant Morris Travers. Krypton like most Noble gases is odourless, colourless and inert. It has the atomic number 36 in the periodic table and belongs in Group 18, the Noble Gases. Krypton (Kr) exists as a colourless, odourless gas and is chemically inert.


 0 kommentar(er)
0 kommentar(er)
